2026年发表文章
1. Wen-Chao Yu, Yan-Lei Yu, Bing-Cheng Dong, Ze-Yu Wang, Au-Qi Du, Song-Wei Li, Buddha Bahadur Basnet, Xiao-Ze Bao, Xuan-Rong Sun, Xing-Nuo Li, Qi Xuan, Qihao Wu*, Hong Wang*, Bin Wei*. Targeted discovery of aromatic glycosides with dual detoxification effects via a highly customized molecular networking platform. Cell Chemical Biology, 2025, 33(1): 132-144. (https://doi.org/10.1016/j.chembiol.2025.12.001) IF:7.2
Abstract

Unlocking bioactive small molecules is essential for drug discovery, but discovering, prioritizing, and characterizing them remains difficult, as they are often found in complex biological extracts. Mass spectrometry-based untargeted metabolomics and MS2 spectral similarity are critical strategies for establishing spectra-inferred structural relationships and discovering unknown metabolites. Current analytical platforms mainly rely on comparing MS2 data using a single algorithm, which often leads to missed annotations of valuable metabolites. MSanalyst addresses this limitation by combining various mass spectral similarity algorithms, thereby enabling more comprehensive metabolite annotation. By applying MSanalyst to a well-studied strain, Kutzneria viridogrisea, we successfully discovered an undescribed class of aromatic glycosides, called kutznaposides. Integrated multi-omics and in vitro experiments further revealed that these molecules are produced through a previously uncharacterized menaquinone shunt pathway. This pathway serves as a crucial defense mechanism, enabling the host organism to eliminate reactive oxygen species and avoid self-toxicity. By demonstrating the strength of algorithmic integration, MSanalyst advances the systematic identification of hidden metabolites, metabolic pathways with essential biological functions, and potential biomedical applications.
2. Lihang Cao, Junjie Ge, Yi Hua, Xiaoze Bao, Zhikun Yang, Hong Wang,* and Hua Chen*. Strain-Release-Driven exo-Cyclization of Bicyclo[1.1.0]butyl Ketones Promoted by Brønsted Acids to Access 2,3-Diazabicyclo[3.1.1]heptenes. Organic Letters, 2026, Doi: 10.1021/acs.orglett.5c04933. (IF: 5.0)
Abstract
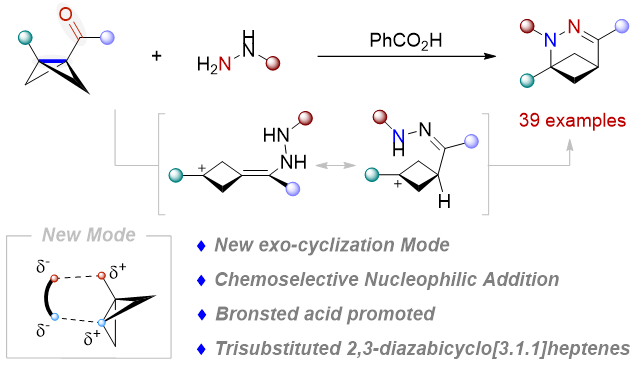
A Brønsted acid-promoted exo-cyclization of bicyclo[1.1.0]butyl ketones with hydrazines has been established. This approach enables efficient ring expansion, leading to a diverse range of 2,3-diazabicyclo[3.1.1]heptenes. Mechanistic studies reveal that the carbonyl group is pivotal, fulfilling a dual role as both the reactive center and a latent activating group. Moreover, the unique dual nucleophilic character of hydrazines, featuring two nitrogen-based nucleophilic sites, is crucial to the success of this transformation.

